- The EC Horizon2020 funded INFRAFRONTIER2020 project (2017 – 2020) supports eligible customers with a free-of-charge COVID-19 Therapeutics Pipeline service implemented as a Trans-national Access activity supporting a total of 5 projects in this call.
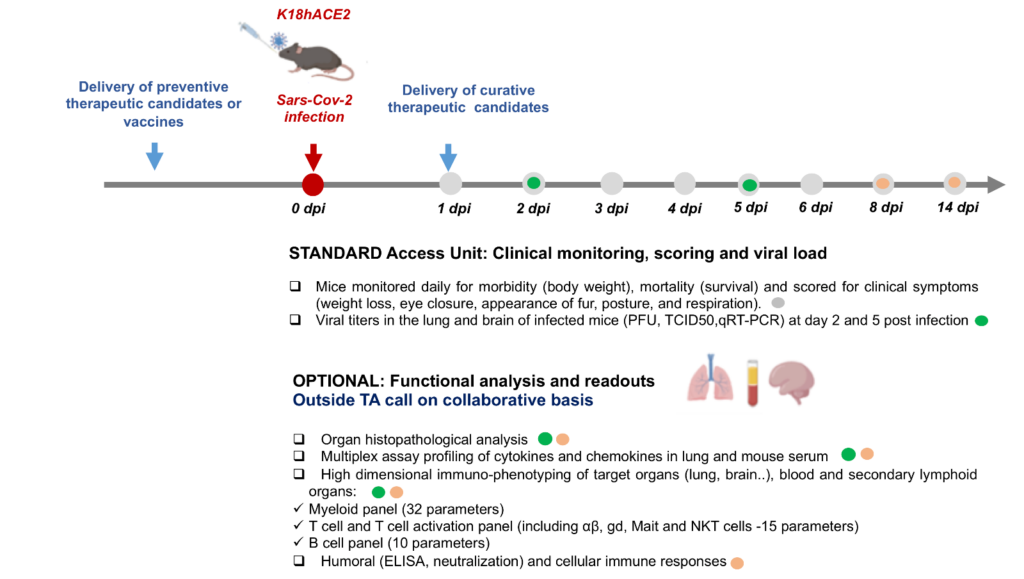
- The access unit offered covers the (1) suitable delivery of preventive therapeutics or vaccines pre-infection or curative therapeutic candidates after infection, (2) infection of COVID-19 mouse models (females) with appropriate SARS-CoV-2 virus titers, (3) monitoring of mice for mortality, morbidity and clinical symptoms and (4) determination of viral titers in the lung and brain.
- Only one access unit per therapeutic candidate will be provided. Additional candidates will require separate independent applications.
- Proposals with customized study designs for the therapeutic pipeline (other than the standard pipeline offered) or requiring any customization of the standard pipeline will be assessed on their technical and financial feasibility by the service provider.
- Support will be provided by the CIPHE experts to analyse and interpret the data.
- A collaboration agreement will be established between applicants and CIPHE/CCP.
- Accepted proposals will start with the provision of the therapeutic candidate that is ready to be injected and end with the delivery of infection data reports to selected applicants.
- Further functional analysis and advanced readouts like organ histopathological analysis, multiplex assay profiling of cytokines and chemokines in lung and mouse serum etc. can be provided on a collaborative basis outside the scope of this TA call.
- A final infection profile report will be provided as a deliverable. The results will be disseminated in the following ways:
- The selected proposals (5 applications, only project descriptions) will be included in a final project report to the EC at the end of 2021.
- The results will be made publicly available 12 months after their submission to the applicant. This can be extended by an additional 6 months under justified circumstances.
- Costs: The access to the INFRAFRONTIER2020 COVID-19 therapeutic pipeline service is free-of-charge. However, the shipment of the therapeutic compound to CIPHE must be borne by the applicants.
- Eligibility: The INFRAFRONTIER2020 Trans-national Access call is open and proposals can be submitted from non-commercial applicants around the world.
- Application: Service requests for the INFRAFRONTIER2020 COVID-19 therapeutic pipeline service can be made via this application form. Applications for the Trans-national Access activity must include a short description of why the therapeutic candidate is a candidate for a COVID-19 vaccine/treatment and future research plans after the INFRAFRONTIER2020 TA service.
- Selection procedure: Proposals from eligible customers for free-of-charge access to the INFRAFRONTIER2020 COVID-19 Therapeutics Pipeline Service will be subject to a review procedure. A mixed panel of INFRAFRONTIER members and an external Evaluation Committee will assess service requests supported by the TA activity. In addition to scientific merit of applicants, relevance and quality of preliminary data, soundness of the proposal and research plans will be assessed. Additionally, experts of CIPHE/CCP will assess the technical feasibility of projects. The technical evaluation of projects may require the provision of additional data.
Applicants will be informed on the outcome of the evaluation within 6 weeks after the end of the call for which the TA application was submitted. All applications will be handled with strict confidentiality.