The expert teams of CIPHE, the Marseille-based INFRAFRONTIER partner coordinating the COVID-19 TA Call, and the contributing partner CCP in Prague are happy about the high value of the submitted projects. Ana Zarubica, CIPHE Deputy Director and the PI supervising the COVID-19 Therapeutics Pipeline, says: “The relevance and quality of all proposals was excellent. They are all addressing fundamental clinical issues.”
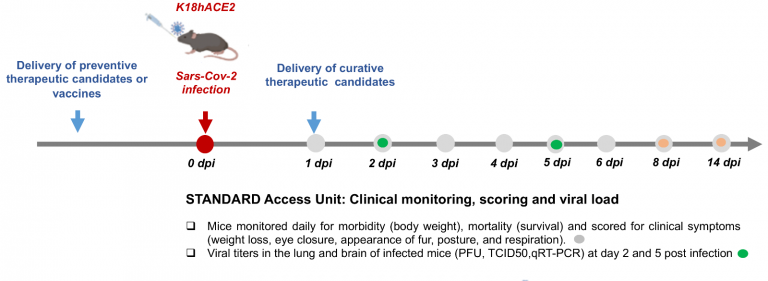
Ana explains why in the end a decisive factor for the selection was the feasibility of the proposals: “Upon infection with SARS-CoV2, the K18-hACE2 mice used in our BSL3 developed a severe disease that results in rapid and high lethality. Therefore they are particularly useful to test vaccines or highly potent treatments blocking virus entry. They are more difficult to use to test anti-inflammatory compounds.”